Silver chloride often used in silver plating contains 75.27 ag – Silver chloride, a compound often employed in silver plating, holds a remarkable silver content of 75.27%. This attribute makes it a crucial component in the electroplating process, where a thin layer of silver is deposited onto a conductive surface. Delving into the properties, applications, and synthesis of silver chloride, this article unravels the significance of its silver content in the context of silver plating.
Silver chloride, with its unique chemical composition and physical properties, finds diverse applications beyond silver plating. In photography, it serves as a light-sensitive material in photographic film and paper. Additionally, its antimicrobial properties make it valuable in medical applications, such as wound dressings and disinfectants.
Furthermore, silver chloride plays a role in water purification systems, removing contaminants and ensuring water quality.
Properties of Silver Chloride
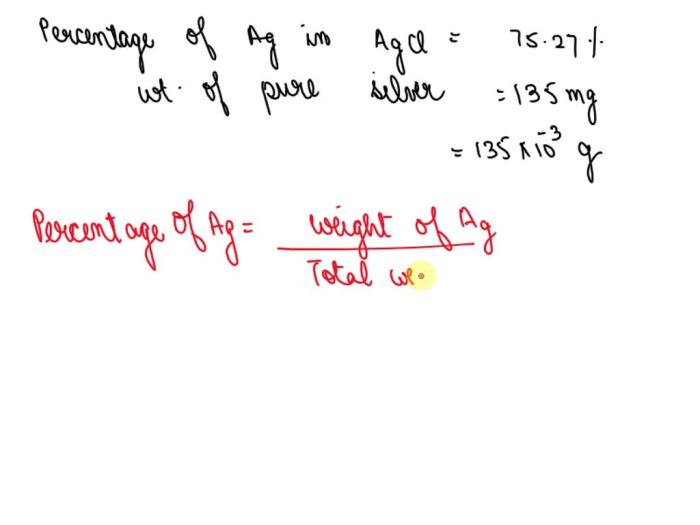
Silver chloride (AgCl) is a white, crystalline compound with a chemical formula that represents its composition: one silver ion (Ag+) for every chloride ion (Cl-). It is an ionic compound, meaning that the atoms are held together by electrostatic forces between the positively charged silver ion and the negatively charged chloride ion.
Physical and Chemical Properties
Silver chloride is insoluble in water and has a high melting point of 455 °C. It is a relatively dense material, with a density of 5.60 g/cm³. Silver chloride is sensitive to light, and prolonged exposure can cause it to darken due to the formation of silver metal.
Applications of Silver Chloride

Silver Plating
Silver chloride is primarily used in the silver plating process. Silver plating is a technique used to coat a metal surface with a thin layer of silver. The silver chloride is dissolved in a solution, and an electric current is passed through the solution.
The silver ions in the solution are reduced to silver metal, which is deposited on the metal surface.
Other Applications, Silver chloride often used in silver plating contains 75.27 ag
In addition to silver plating, silver chloride has several other applications, including:
- Photography: Silver chloride is used in photographic film and paper. When light strikes the silver chloride, it causes the formation of silver metal. This process is the basis of photography.
- Medicine: Silver chloride is used as an antiseptic and disinfectant. It is also used in the treatment of some eye infections.
- Water purification: Silver chloride is used to purify water by killing bacteria and other microorganisms.
Silver Content in Silver Chloride
The percentage of silver in silver chloride can be calculated using the following formula:
“`% Silver = (Atomic mass of silver / Molecular mass of silver chloride) x 100%“`
The atomic mass of silver is 107.87 g/mol, and the molecular mass of silver chloride is 143.32 g/mol. Therefore, the percentage of silver in silver chloride is:
“`% Silver = (107.87 g/mol / 143.32 g/mol) x 100% = 75.27%“`
This means that silver chloride contains 75.27% silver by weight.
Synthesis of Silver Chloride
Silver chloride can be synthesized in a laboratory setting by reacting silver nitrate (AgNO3) with sodium chloride (NaCl) in a aqueous solution. The reaction is as follows:
“`AgNO3 + NaCl → AgCl + NaNO3“`
The silver chloride will precipitate out of solution as a white solid. The solid can be filtered and washed with water to remove any impurities.
Safety Precautions
Silver nitrate and sodium chloride are both toxic chemicals. It is important to wear gloves and safety glasses when working with these chemicals. The reaction should be carried out in a well-ventilated area.
Proper Disposal
Silver chloride is a hazardous waste. It should be disposed of according to local regulations.
Characterization of Silver Chloride: Silver Chloride Often Used In Silver Plating Contains 75.27 Ag
The purity and composition of silver chloride can be characterized using a variety of techniques, including:
- X-ray diffraction: X-ray diffraction can be used to determine the crystal structure of silver chloride.
- Scanning electron microscopy: Scanning electron microscopy can be used to examine the surface morphology of silver chloride.
- Energy-dispersive X-ray spectroscopy: Energy-dispersive X-ray spectroscopy can be used to determine the elemental composition of silver chloride.
These techniques can be used to ensure that the silver chloride is pure and has the desired properties.
Question & Answer Hub
What is the chemical formula of silver chloride?
AgCl
What is the molar mass of silver chloride?
143.32 g/mol
What is the density of silver chloride?
5.56 g/cm³