Embark on an enlightening journey with Student Exploration Electron Configuration Gizmo Answers, an indispensable resource that unlocks the complexities of electron configuration. This comprehensive guide empowers learners to delve into the fundamental principles governing the arrangement of electrons within atoms, laying the foundation for a deeper understanding of chemical bonding and reactivity.
Through a series of interactive simulations and engaging explanations, the Gizmo fosters a hands-on approach to exploring electron configurations. By manipulating the Gizmo’s intuitive interface, students can visualize the distribution of electrons in various elements, observe the patterns and trends that emerge, and gain insights into the exceptions and special cases that challenge the Aufbau principle.
Electron Configuration Gizmo Introduction
The Electron Configuration Gizmo is an interactive simulation that allows students to explore the electron configurations of elements. It is designed to help students understand the fundamental concepts of electron configuration, including the Aufbau principle, the relationship between electron configuration and the Periodic Table, and the role of electron configuration in determining chemical properties.
The Gizmo provides a visual representation of the electron configuration of an element, showing the number of electrons in each energy level and subshell. Students can manipulate the Gizmo to change the element and observe the changes in its electron configuration.
They can also add or remove electrons to see how this affects the element’s electron configuration.
Elements and Electron Configuration
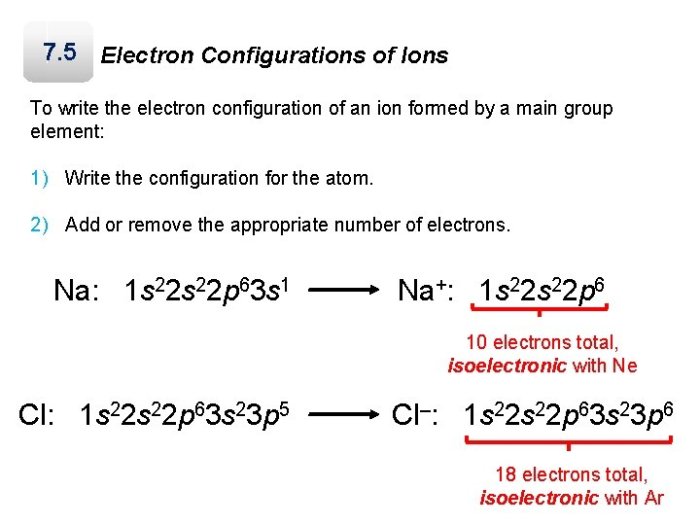
The electron configuration of an element is the distribution of its electrons across its energy levels and subshells. The number of electrons in each energy level is determined by the element’s atomic number. The electron configuration of an element can be predicted using the Aufbau principle, which states that electrons fill the lowest energy levels first.
| Element | Electron Configuration |
|---|---|
| Hydrogen | 1s1 |
| Helium | 1s2 |
| Lithium | 1s22s1 |
| Beryllium | 1s22s2 |
| Boron | 1s22s22p1 |
The electron configuration of an element is closely related to its position on the Periodic Table. Elements in the same group (vertical column) have the same number of valence electrons, which are the electrons in the outermost energy level. Valence electrons are responsible for chemical bonding, so elements in the same group have similar chemical properties.
Manipulating the Gizmo

To use the Electron Configuration Gizmo, select an element from the drop-down menu. The Gizmo will display the element’s electron configuration in a visual representation. You can add or remove electrons by clicking on the plus or minus buttons. You can also use the “Show Orbitals” feature to visualize the distribution of electrons in the element’s orbitals.
By manipulating the Gizmo, you can explore the electron configurations of different elements and see how they change when electrons are added or removed. This can help you to understand the Aufbau principle and the relationship between electron configuration and the Periodic Table.
Patterns and Trends
There are several patterns and trends in electron configurations that can be observed using the Electron Configuration Gizmo. One pattern is that the number of electrons in each energy level increases as you move down the Periodic Table. Another pattern is that the electron configurations of elements in the same group are similar.
This is because elements in the same group have the same number of valence electrons.
The electron configuration of an element can also be used to predict its chemical properties. For example, elements with a full valence shell are unreactive, while elements with one or two valence electrons are highly reactive. This is because elements with a full valence shell are stable, while elements with one or two valence electrons are not.
Exceptions and Special Cases
There are some exceptions to the Aufbau principle. For example, the electron configuration of chromium is [Ar]3d 54s 1, which violates the Aufbau principle because the 4s subshell is filled before the 3d subshell. This is due to the fact that the 3d subshell is more stable than the 4s subshell.
There are also some special cases where the electron configuration of an element cannot be predicted using the Aufbau principle. For example, the electron configuration of copper is [Ar]3d 104s 1, which has a full 3d subshell. This is due to the fact that the 3d subshell is more stable than the 4s subshell.
Applications of Electron Configuration: Student Exploration Electron Configuration Gizmo Answers
Electron configuration is used in a variety of scientific fields, including chemistry, physics, and materials science. In chemistry, electron configuration is used to predict chemical bonding and reactivity. In physics, electron configuration is used to explain the properties of atoms and molecules.
In materials science, electron configuration is used to design new materials with specific properties.
Electron configuration is a fundamental concept in chemistry and physics. It is used to explain a wide range of chemical and physical phenomena. By understanding electron configuration, you can gain a deeper understanding of the world around you.
FAQ Section
What is the purpose of the Electron Configuration Gizmo?
The Electron Configuration Gizmo is designed to help students visualize and understand the electron configurations of various elements. It allows learners to manipulate the number of electrons and observe the resulting changes in electron distribution.
How can I use the Gizmo to determine the electron configuration of an element?
To determine the electron configuration of an element using the Gizmo, select the element from the periodic table and observe the electron distribution displayed in the simulation. The Gizmo also provides a table of electron configurations for quick reference.
What are some of the patterns and trends in electron configurations?
Electron configurations exhibit patterns and trends based on the element’s position on the periodic table. These patterns include the filling of electron shells and subshells, as well as the relationship between electron configuration and chemical properties.